microDOAC
A Point-of-Care analyzer for the semi-quantitative analysis of direct oral anticoagulant (DOAC) concentration.
microDOAC is designed to be the first POC device covering the real clinical need for evaluation of DOAC patients in fresh whole blood in a matter of minutes. microDOAC will offer a key benefit for clinicians where routine laboratory tests may fail to detect DOAC levels, such as apixaban (Eliquis ®). DOAC adverse effects have increased due to a higher volume of prescription of these drugs.
The main benefits that make microDOAC unique are:
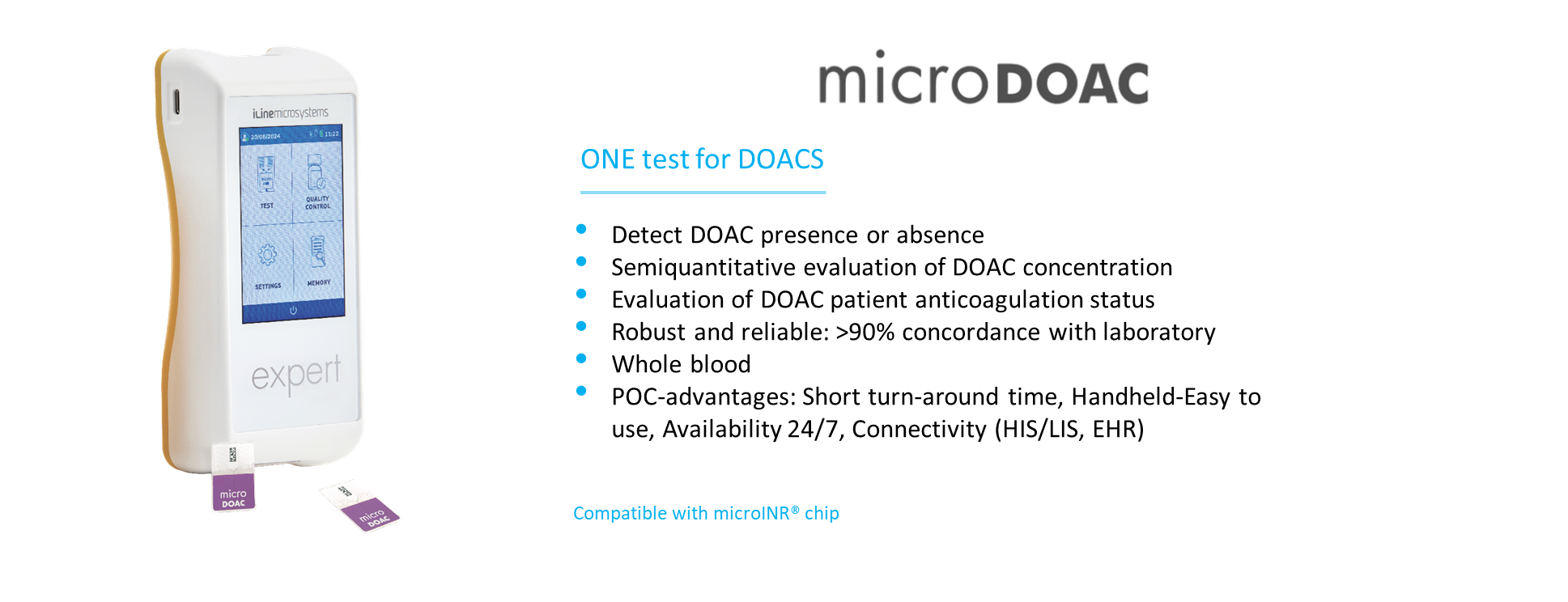
• Detection of DOAC presence or absence in a whole blood single test.
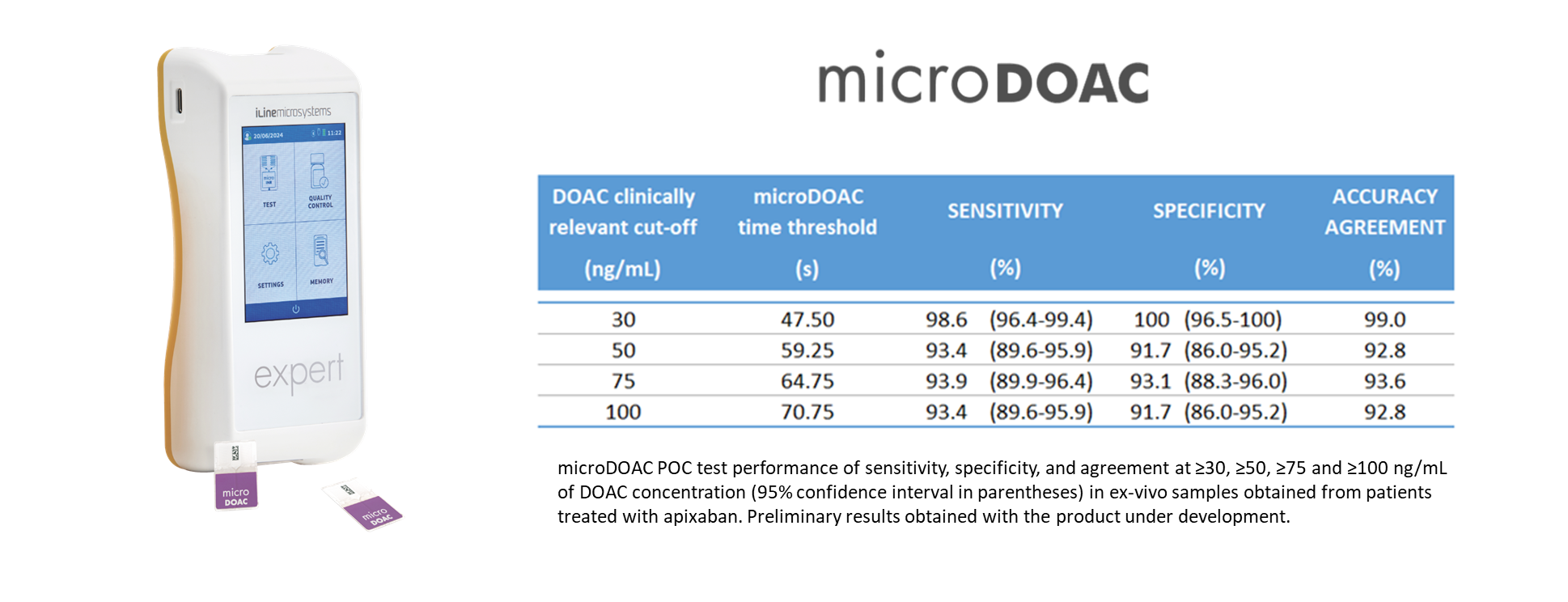
• Semiquantitative evaluation of DOAC concentration at clinically relevant thresholds such as 30, 50 or 100 ng/mL.
• Evaluation of DOAC patients’ anticoagulation status and pharmacodynamic response.
• Robust and reliable: higher than 90 % sensitivity and specificity at selected clinically relevant thresholds.
• Easy to use and handheld.
• Both capillary and venous blood.
• Professional platform with wireless connectivity (Wi-Fi, Bluetooth), Ethernet, and easy HIS/LIS integration.
Over the last years, the company has been working on a microDOAC Chip project aimed at incorporating it into the Expert meter to create a multiTEST system for both microINR and microDOAC tests. Both PT/INR and microDOAC tests enable informed decisions, saving vital time in oral anticoagulation therapies.
The microDOAC system will support better decision-making than laboratory tests, due to the accessibility (24/7) and short turnaround time (less than 2 minutes); therefore, it has the potential to improve patient outcomes in both emergency and routine care settings.
The microDOAC system will allow an earlier intervention and better management of anticoagulated patients, providing a more effective treatment in the following situations:
1. Emergency situations with a major bleeding.
2. Prior to an urgent procedure with high bleeding risk.
3. Management of reversal procedures.
4. Stroke patients before a thrombolysis intervention.
5. Patient follow-up: adherence, special DOAC population, drug interactions...
Universally available for use across a wide range of settings: Emergency Department, ICUs, ORs, Ambulances, Anticoagulation Clinics, Primary Care (physician offices, physician office labs) and Cardiology visits.
Publications:
1.Cuker et al. 2023 ‘A novel Point-of-Care whole blood portable analyser for direct oral anticoagulant monitoring’. Research and Practice in Thrombosis and Haemostasis Vol. 7 Supplement. Oral Communication at ISTH 2023 Congress in Montreal, earning recognition as one of the conference's "Best sessions ISTH2023”.
2. ‘Novel Point-of-Care (POC) platform for direct oral anticoagulants (DOAC) and vitamin K antagonists (VKA) testing: microDOAC and microINR assays’, selected as an in-person poster presentation at THSNA 2024 in Chicago, IL. Pending to be published in the American Journal of Hematology. https://www.thsna.org/online-admin/Abstract-All.php
3. Cuker et al. 2024. ‘A Point-of-Care analyzer (microDOAC) for the semi-quantitative analysis of direct oral anticoagulant (DOAC) concentration: a pilot study of apixaban-treated patients’, oral communication at the ISTH 2024 Congress in Bangkok. Oral Communication Abstracts, Research and Practice in Thrombosis and Haemostasis, Volume 8, Supplement 2.
microINR Expert is not available in the US. microDOAC is a product under development.