You are visiting the Global Version of iLine Microsystems website. This version does not correspond to the US website
What is the microlNR System?
The microINR System consists of a portable analyzer (or Meter) and disposable test strips (or Chips). The microINR Chips are intended to be used exclusively with the microINR and microINR Link Meters
Which is the microlNR intended use?
The microINR system is intended to monitor oral anticoagulation therapy (OAT) with vitamin K antagonist drugs. The microINR system determines quantitative prothrombin time (PT) in INR (International Normalized Ratio) units with fresh capillary blood performed by fingersticking. The microINR system is a medical device for in-vitro diagnostics intended for professional and self-testing use.
What is the Oral Anticoagulant Therapy (OAT)?
The indication of Oral Anticoagulation Treatment (OAT) is the prevention of cardiovascular events secondary to thromboembolic processes. Under normal physiological conditions, coagulation is not activated in the bloodstream. However, when a vascular injury occurs, both internal and external, blood clotting mechanism is activated. Coagulation system avoids massive blood losses, hemorrhages and its consequences.
Treatment features:
- OAT is effective as thromboembolic disease treatment and increases blood clotting time. Once the patient is treated, if clotting time does not increase sufficiently; it means there is not enough protective effect of the drug on the patient. On the contrary, if the clotting time is too high, hemorrhage risk appears. In order to avoid too high or low clotting time values, this clotting time value has to be monitored, analyzing patient blood samples. If it lies within acceptable values, therapy is safe and effective.
- The dosage of the treatment must be established by a physician. The dosage must be adjusted individually for each patient after analyzing test results and never should be calculated by weight, age…
- The drug is administrated by oral route, and it is absorbed through the gastrointestinal tract before it reaches the bloodstream. Any dysfunction of the digestive tract can affect directly to the absorption of the drug and can alter its levels on blood. OAT exerts its pharmacological effect on liver as it blocks the proper synthesis of coagulation factors.
- The combined administration of some drugs can boost the effect of OAT, in other cases the OAT effect can be diminished.
What is the INR and what is it used for?
Variations on the obtained measures exist between different PT measuring devices due to the nature of the reagents of the thromboplastin or the equipment used.
In order to compare different measurement methods, the WHO recommended in 1977 a method of standardization of systems. The prothrombin time values are converted to INR, International Normalized Ratio (INR). The INR is the ratio of a patient's prothrombin time to a normal (control) sample, raised to the power of the ISI value for the analytical system being used.
The INR is use for the patient´s monitoring under oral anticoagulant therapy (OAT).
Which is the microlNR measurement principle?
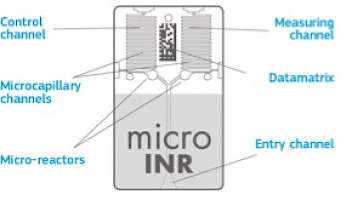
The technology used by the microINR System is based on the microfluidics of the microINR Chip, which allows storing, dosing, moving and/or mixing small volumes of liquids to perform a chemical reaction.The microINR Chips contain two channels, one for measurement and the other for control.
Each channel consists of a micro-reactor that contains the reagent and a microcapillary where the INR is determined. The reagent used in the measuring channel contains human recombinant thromboplastin and the reagent in the control channel contains recombinant thromboplastin and human coagulation factors to normalize the patient’s blood. The blood is applied to the Chip through the entry channel,separated into two channels and mixed with the reagents contained in each micro-reactor. The coagulation cascade is triggered instantly. When clotting, blood undergoes a sudden change on its flow rate behavior, which is detected by an embedded Machine Vision System (MVS) and a proprietary algorithm. The measured prothrombin time is converted into INR using lot-specific calibration parameters microprinted on the Chip datamatrix.
Which is the reagent type and which are its features?
The reagent used on the microINR Chips is a human recombinant thromboplastin with an ISI of approximately 1, and it is highly sensitive to the vitamin K synthesis-dependent factors (II, VII, IX y X).
Which is the correct sample application technique?
You can find the sample application technique description in the Instructions for Use (Section 3). We strongly recommend you watching the microINR Training Video, which is allocated in this website (Resources).
Which is the accuracy and precision of the microINR?
You can find the accuracy and precision data of the System in the microINR Chips Instructions for Use (Section: Specific Test Performance Data)
How is the microINR calibrated?
Each batch of microINR Chips has been calibrated against a reference batch of human recombinant thromboplastin traced to International Reference Thromboplastin of the World Health Organization. These calibration values (ISI and MNPT) are encoded in the printed Datamatrix of each microINR Chip. Therefore, every test is automatically and individually calibrated eliminating any risk of human error.
What should I do when an unexpected result is obtained?
Please check the Instructions for Use and make sure all the important steps for a correct sample application have been followed. If an unexpected result is obtained, please contact your healthcare provider and follow their instructions. In case you are a healthcare professional, please contact your distributor/provider.